Home /
Expert Answers /
Chemistry /
this-is-everything-nbsp-gibbs-energy-and-the-equilibrium-constant-for-the-reaction-mathrm-pbo-pa989
(Solved): this is everything Gibbs energy and the equilibrium constant for the reaction \( \mathrm{PbO} ...

this is everything
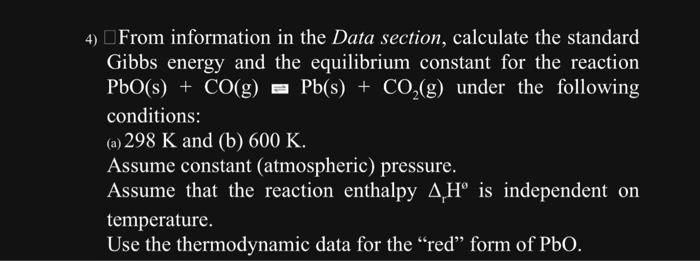

Gibbs energy and the equilibrium constant for the reaction \( \mathrm{PbO}(\mathrm{s})+\mathrm{CO}(\mathrm{g}) \) Pb(s) \( +\mathrm{CO}_{2}(\mathrm{~g}) \) under the following conditions: (a) \( 298 \mathrm{~K} \) and (b) \( 600 \mathrm{~K} \). Assume constant (atmospheric) pressure. Assume that the reaction enthalpy \( \Delta_{\mathrm{r}} \mathrm{H}^{\circ} \) is independent on temperature. Use the thermodynamic data for the "red" form of PbO.
4) \( \square \) From information in the Data section, calculate the standard Gibbs energy and the equilibrium constant for the reaction \( \mathrm{PbO}(\mathrm{s})+\mathrm{CO}(\mathrm{g}) \equiv \mathrm{Pb}(\mathrm{s})+\mathrm{CO}_{2}(\mathrm{~g}) \) under the following conditions: (a) \( 298 \mathrm{~K} \) and (b) \( 600 \mathrm{~K} \). Assume constant (atmospheric) pressure. Assume that the reaction enthalpy \( \Delta_{r} H^{\circ} \) is independent on temperature. Use the thermodynamic data for the "red" form of \( \mathrm{PbO} \).