Home /
Expert Answers /
Chemistry /
this-is-one-question-pease-help-suppose-you-have-two-identical-balloons-you-place-1-mol-of-neon-in-pa538
(Solved): this is one question, pease help Suppose you have two identical balloons. You place 1 mol of neon in ...


this is one question, pease help
Suppose you have two identical balloons. You place 1 mol of neon in one balloon; you place \( 1 \mathrm{~mol} \) of argon in the other. Which balloon has the greater volume? Choose one: A. both talloons lisve the same volume B. the balloce containing argon C. the balloon containititg veou
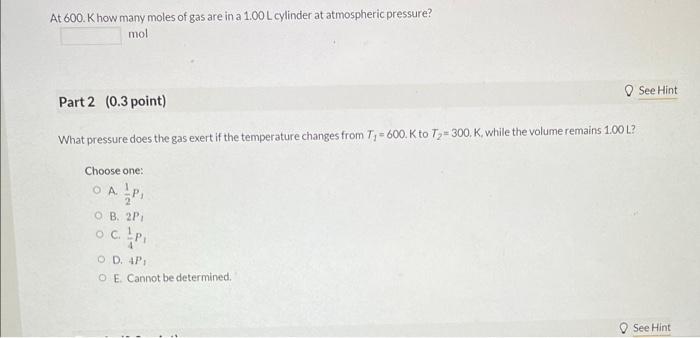
At 600 . Khow many moles of gas are in a \( 1.00 \mathrm{~L} \) cylinder at atmospheric pressure? mol Part 2 ( \( 0.3 \) point) What pressure does the gas exert if the temperature changes from \( T_{1}=600 . \mathrm{K} \) to \( T_{2}=300, \mathrm{~K} \), while the volume remains \( 1.00 \mathrm{~L} \) ? Choose one: A. \( \frac{1}{2} P_{1} \) B. \( 2 P_{1} \) C. \( \frac{1}{4} P_{1} \) D. \( +P_{1} \) E. Cannot be determined.
If the pressure of the gas changes from \( 0.50 \mathrm{~atm} \) to \( 1.00 \mathrm{~atm} \) while the temperature is maintained at \( 300 . \mathrm{K} \), what happens to the volume occupied by the gas? Choose one: A. It increases by a factor of 2 . B. It decreases by a factor of 2 . C. It remains constant. D. There is no way to tell.
Expert Answer
1. 1 mole of neon and 1 mole of argon has same volume Ans. A. both ballon have the same volume 2. Given Temperature (T) = 600 K Volume (V) = 1 L Pressure (P) = 1 at