Home /
Expert Answers /
Chemistry /
under-certain-conditions-the-rate-of-this-reaction-is-zero-order-in-ammonia-with-a-rate-constant-of-pa597
(Solved): Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of ...
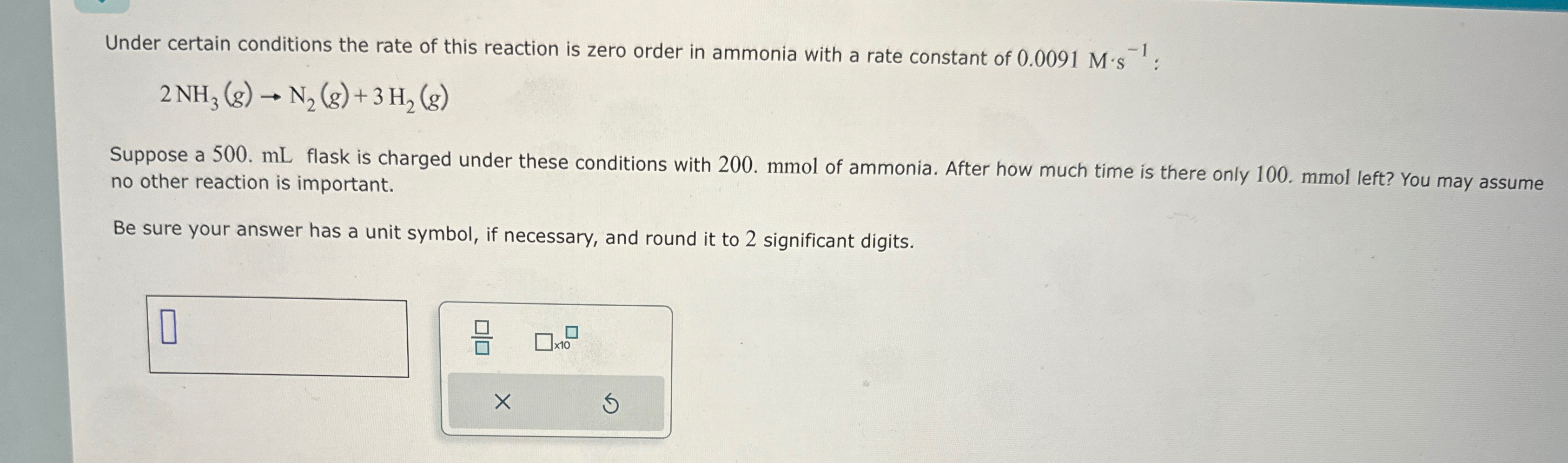
Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of
0.0091M*s^(-1):
2NH_(3)(g)->N_(2)(g)+3H_(2)(g)Suppose a 500.
mLflask is charged under these conditions with 200. mmol of ammonia. After how much time is there only 100. mmol left? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.