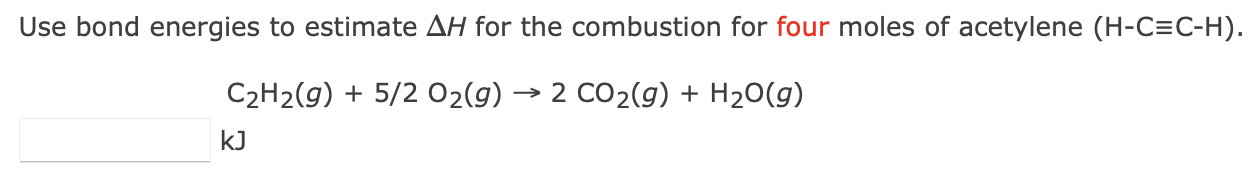Home /
Expert Answers /
Chemistry /
use-bond-energies-to-estimate-delta-h-for-the-combustion-for-four-moles-of-acetylene-m-pa575
(Solved): Use bond energies to estimate \( \Delta H \) for the combustion for four moles of acetylene \( (\m ...
Use bond energies to estimate \( \Delta H \) for the combustion for four moles of acetylene \( (\mathrm{H}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H}) \). \[ \underset{k J}{\mathrm{C}_{2} \mathrm{H}_{2}(g)+5 / 2 \mathrm{O}_{2}(g) \rightarrow 2 \mathrm{CO}_{2}(g)+\mathrm{H}_{2} \mathrm{O}(g)} \]
Expert Answer
Reaction is C2H2(g) + 5/2O2(g) 2CO2(g) + H2O(g) type of bond Bond energy Number of bond in reactant Number of bond in product C - H 413 KJ/mol 2 0 C C 839 KJ/mol 1 0 O