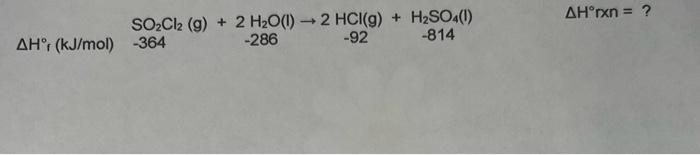Home /
Expert Answers /
Chemistry /
use-the-ah-values-provided-to-calculate-the-ahr-for-the-following-reaction-hr-kj-mol-pa327
Expert Answer
The enthalpy of a reaction is equal to the difference of the formation enthalpies of the products and the reactants. The sum of the formation enthalpies of the products i.e.