Home /
Expert Answers /
Chemistry /
use-the-references-to-access-important-values-if-needed-for-this-question-the-pauli-exclusion-princ-pa145
(Solved): Use the References to access important values if needed for this question. The Pauli Exclusion Princ ...
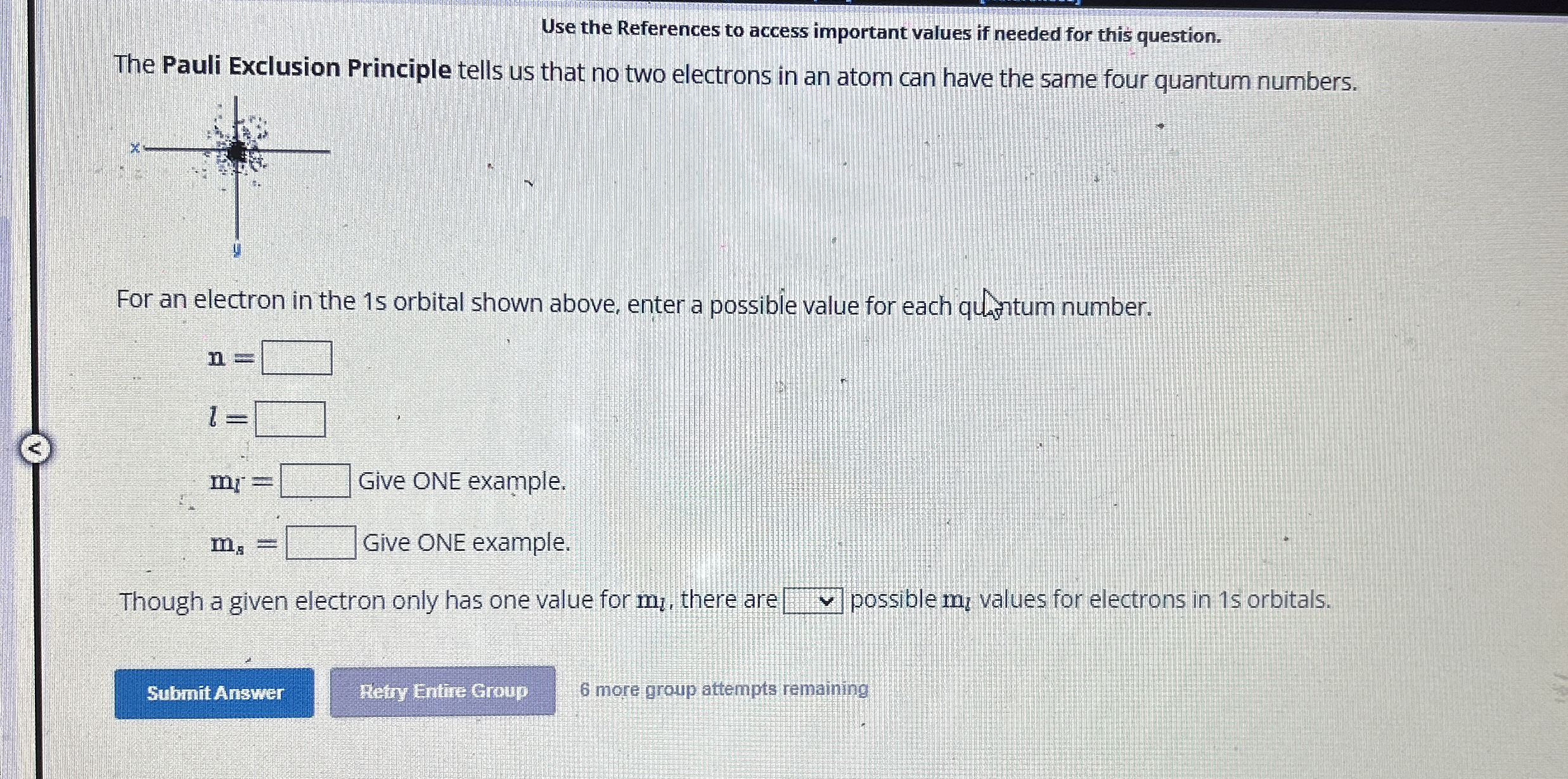
Use the References to access important values if needed for this question. The Pauli Exclusion Principle tells us that no two electrons in an atom can have the same four quantum numbers. For an electron in the 15 orbital shown above, enter a possible value for each quantum number.
n=
l=
m_(l)= Give ONE example.
m_(s)= Give ONE example. Give ONE example. Give ONE example. Though a given electron only has one value for
m_(l), there are
◻possible
m_(l)values for electrons in
1sorbitals. 6 more group attempts remaining