Home /
Expert Answers /
Chemistry /
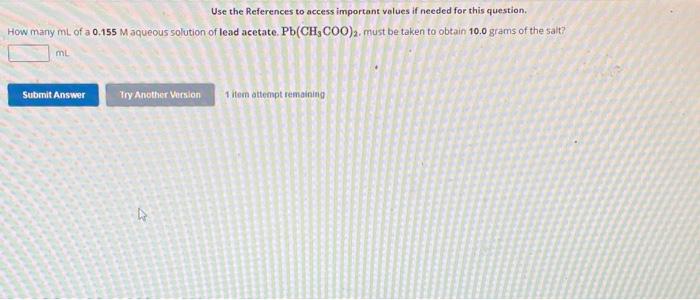
use-the-references-to-access-important-volues-if-needed-for-this-question-how-many-ml-of-a-0-155m-pa489
Expert Answer
Given data:Molarity of lead acetate solution