Home /
Expert Answers /
Chemistry /
using-calculus-you-can-obtain-the-folloming-relationship-betueen-the-concentration-of-mathrm-a-pa987
(Solved): Using calculus, you can obtain the folloming relationship betueen the concentration of \( \mathrm{A ...
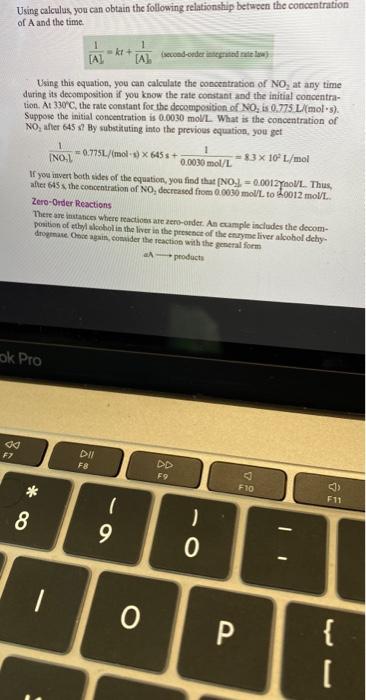
Using calculus, you can obtain the folloming relationship betueen the concentration of \( \mathrm{A} \) and the time. \[ \frac{1}{[\mathrm{~A}]}=k t+\frac{1}{[\mathrm{~A}]} \text { (sowad-onder initguinad mition) } \] Using this equation, you can calculate the conccntration of \( \mathrm{NO}_{2} \) at any time during its desomposition if you know the rate constant and the initial concentration. At 330 C, the rate constant for the decomposition of \( \mathrm{NO}_{2} \) is \( 0.775 \mathrm{~L} \) (mol \( \left./ \mathrm{s}\right) \). Supposo the initial concentration is \( 0.0030 \mathrm{~mol} / \mathrm{L} \). What is the concentration of \( \mathrm{NO}_{2} \) after \( 645 \mathrm{~s} \) ? By substituting into the previous equation, you get \[ \frac{1}{\mathrm{INO}, \mathrm{l}}=0.775 \mathrm{~L} /(\mathrm{mol} \cdot \mathrm{s}) \times 645 \mathrm{~s}+\frac{1}{0.0030 \mathrm{~mol} / \mathrm{L}}=83 \times 10^{2} \mathrm{~L} / \mathrm{mol} \] If you illvert both eider of the equation, you find that \( \left[\mathrm{NO}_{3}\right]=0.0012 \mathrm{Y}_{201 / 1} \). Thus, alter 645 \( \mathrm{x} \), the concentration of \( \mathrm{NO}_{2} \) decresed from \( 0.0030 \mathrm{~mol} \mathrm{~L} \) to \( \{0012 \) mol/L. Zero-Order Reactions These are intanco Whicre reactions are zen-order. As comple iscludes the decomposition of etby alocholia the lintr in the preseact of the enyyme liver alcobol dehydivermane Omic again, fostuder the reaction with the general form