Home /
Expert Answers /
Chemistry /
using-the-phase-diagram-determine-the-molar-composition-of-the-vapor-in-equilibrium-with-a-boiling-pa481
(Solved): Using the phase diagram, determine the molar composition of the vapor in equilibrium with a boiling ...
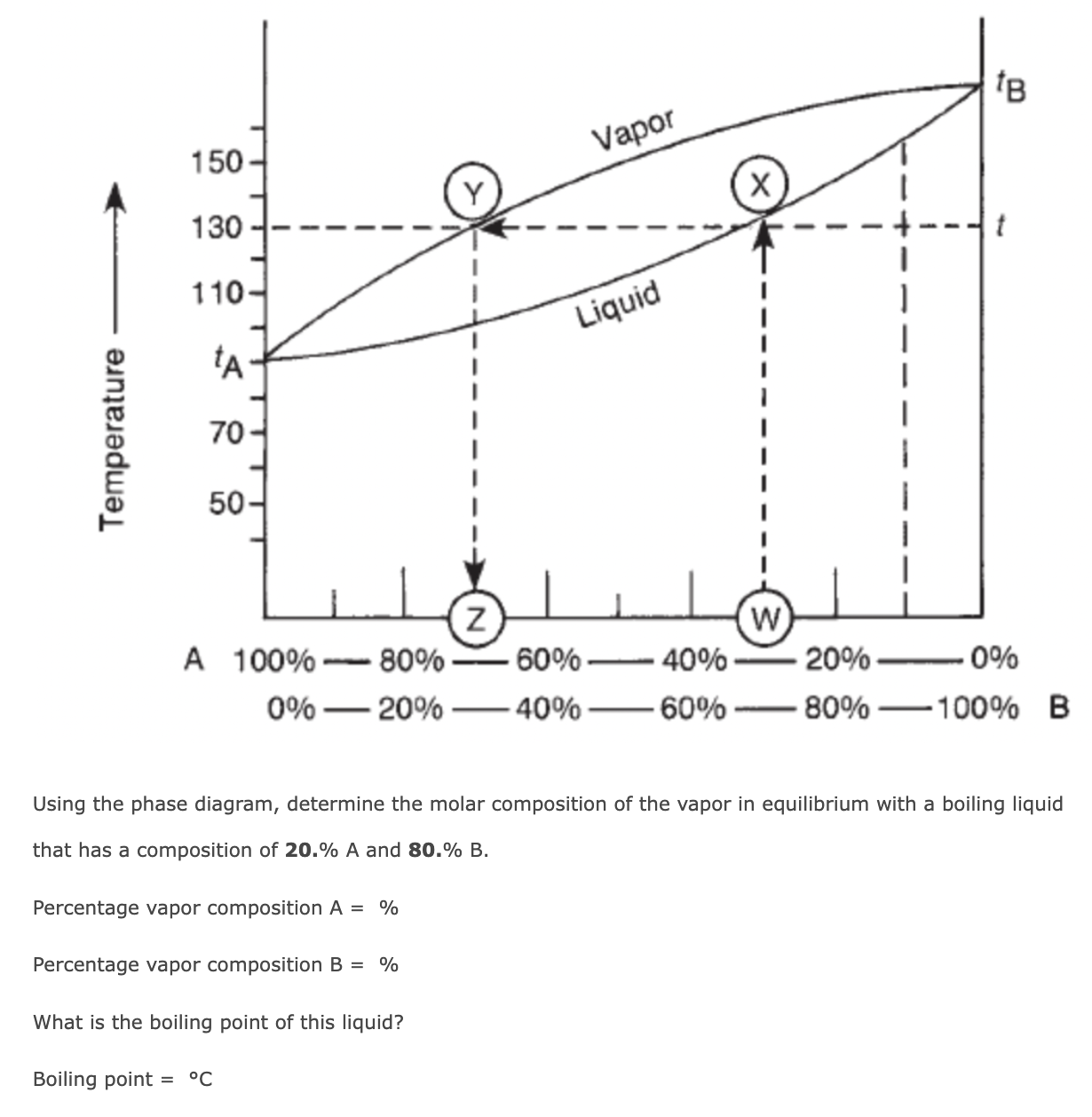
Using the phase diagram, determine the molar composition of the vapor in equilibrium with a boiling liquid that has a composition of
20.%Aand
80.%B. Percentage vapor composition
A=%Percentage vapor composition
B=%What is the boiling point of this liquid? Boiling point
=\deg C