Home /
Expert Answers /
Chemistry /
using-the-same-approach-calculate-how-many-milliliters-of-0-237-m-naoh-are-needed-to-completely-ne-pa136
(Solved): Using the same approach calculate how many milliliters of 0.237 M NaOH are needed to completely ne ...
Using the same approach calculate how many milliliters of 0.237 M NaOH are needed to completely neutralize 25.7 mL of 0.308 M H2C4H4O6.
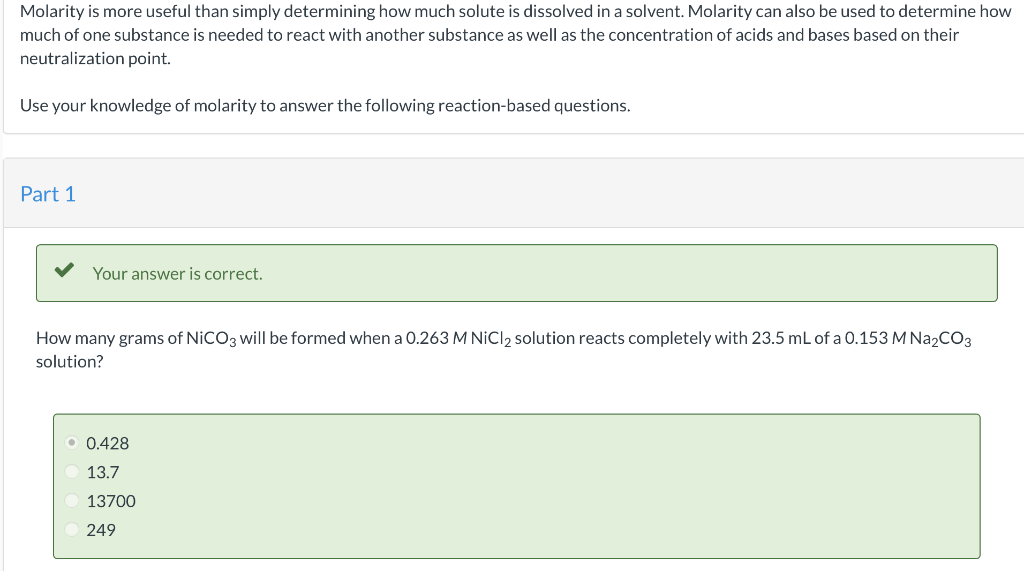
Molarity is more useful than simply determining how much solute is dissolved in a solvent. Molarity can also be used to determine how much of one substance is needed to react with another substance as well as the concentration of acids and bases based on their neutralization point. Use your knowledge of molarity to answer the following reaction-based questions. Part 1 Your answer is correct. How many grams of \( \mathrm{NiCO}_{3} \) will be formed when a \( 0.263 \mathrm{M} \mathrm{NiCl}_{2} \) solution reacts completely with \( 23.5 \mathrm{~mL} \) of a \( 0.153 \mathrm{M} \mathrm{Na}_{2} \mathrm{CO}_{3} \) solution? \( 0.428 \) \( 13.7 \) 13700 249
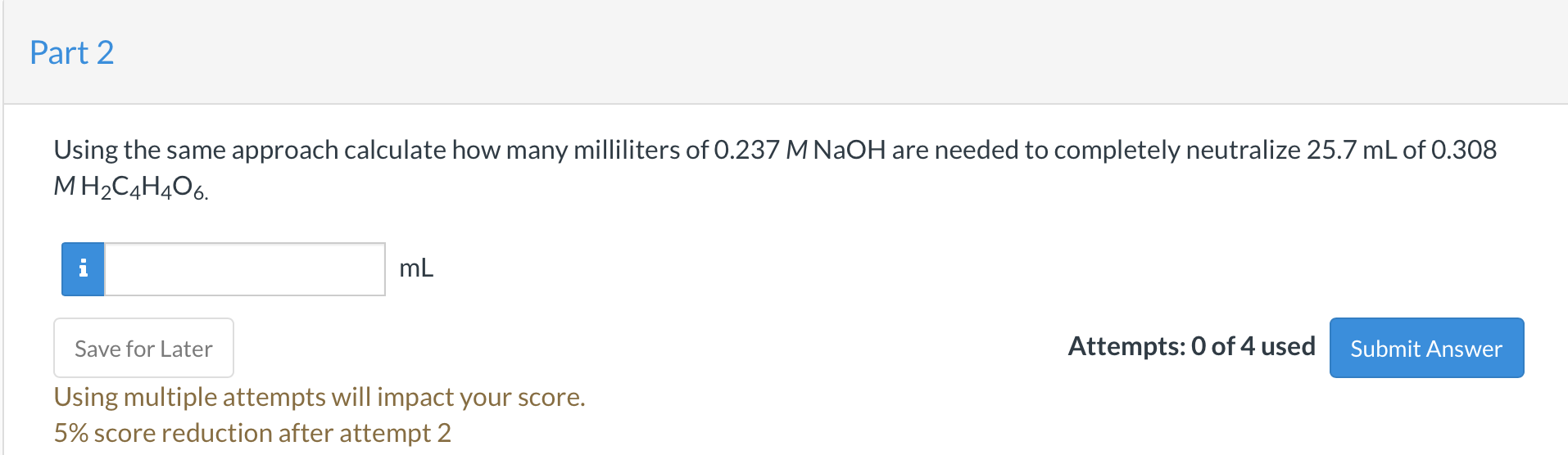
Using the same approach calculate how many milliliters of \( 0.237 \mathrm{MNaOH} \) are needed to completely neutralize \( 25.7 \mathrm{~mL} \) of \( 0.308 \) \( \mathrm{M} \mathrm{H}_{2} \mathrm{C}_{4} \mathrm{H}_{4} \mathrm{O}_{6} \). \( \mathrm{mL} \) Attempts: 0 of 4 used Using multiple attempts will impact your score. \( 5 \% \) score reduction after attempt 2