Home /
Expert Answers /
Chemistry /
water-interacts-with-polar-substances-like-oh-groups-but-not-with-non-polar-sibutances-like-methy-pa363
(Solved): Water interacts with polar substances like --OH groups but not with non-polar sibutances like methy ...
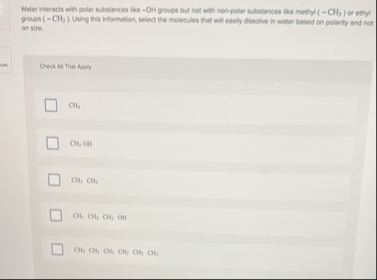
Water interacts with polar substances like --OH groups but not with non-polar sibutances like methy (
-CH_(3)) or ethyl groups (
=CH_(2)) . Using this information, select the molecules that will easily dissolve in weter based on polarity and not on site. Check AI Thei Apely
◻
CH_(4)
◻Clis, OH
◻
CH,CH
◻
CH_(2)CH_(2)CH_(4)CH
◻
Cl_(2)CH_(1)Cl_(3)Cl_(2)CH_(2)Cl_(4)