Home /
Expert Answers /
Chemistry /
what-intermolecular-forces-are-present-in-pure-samples-of-both-ch3ch2oh-and-ch3ch2nh2-pa867
(Solved): What intermolecular forces are present in pure samples of both CH3CH2OH and CH3CH2NH2 ...


What intermolecular forces are present in pure samples of both and ? A) Dispersion forces, dipole-dipole forces, and hydrogen bonding.
What intermolecular forces are present between two molecules of ?
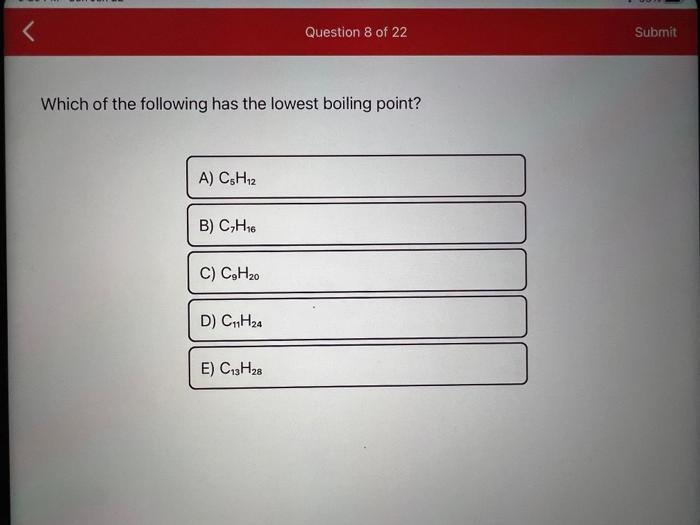
Which of the following has the lowest boiling point?
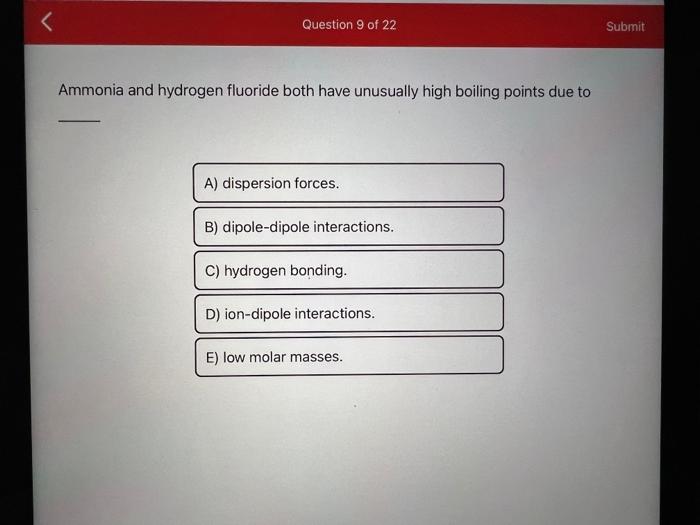
Ammonia and hydrogen fluoride both have unusually high boiling points due to
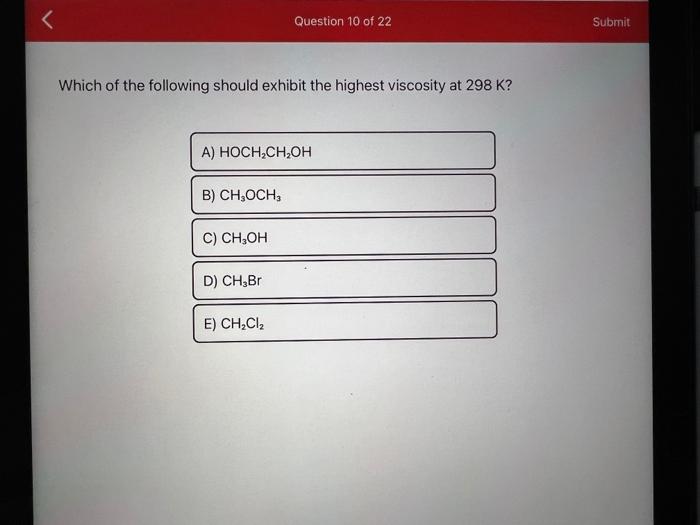
Which of the following should exhibit the highest viscosity at ?
Expert Answer
Answer1)option(A)Dispersion forces, dipole-dipole interaction and H- bondingSince these molecules are polar molecules, they will interact with other p