Home /
Expert Answers /
Chemistry /
what-is-the-calculated-value-of-the-cell-potential-at-298k-for-an-electrochemical-cell-with-the-fol-pa864
(Solved): What is the calculated value of the cell potential at 298K for an electrochemical cell with the fol ...
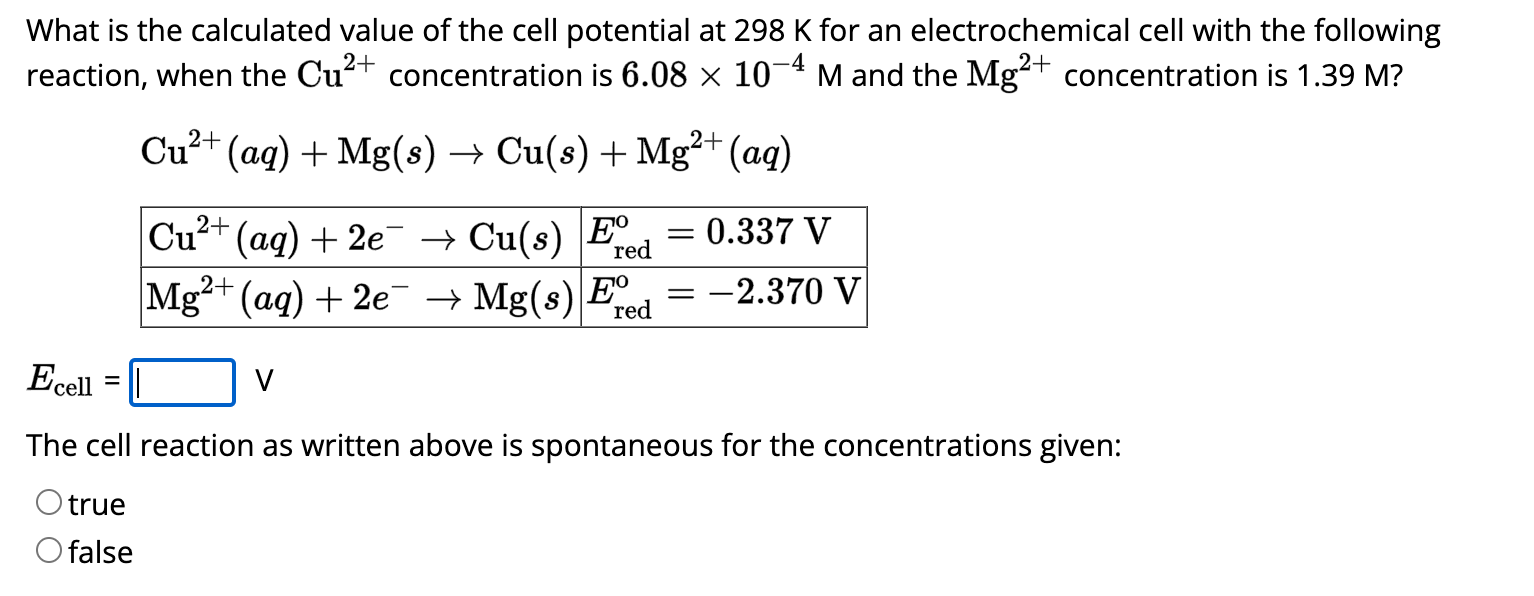
What is the calculated value of the cell potential at
298Kfor an electrochemical cell with the following reaction, when the
Cu^(2+)concentration is
6.08\times 10^(-4)Mand the
Mg^(2+)concentration is
1.39M?
[Cu^(2+)(aq)+Mg(s)->Cu(s)+Mg^(2+)(aq)],[{(:[Cu^(2+)(aq)+2e^(-)->Cu(s),E_(red )^(o)=0.337V]),(Mg^(2+)(aq)+2e^(-)->Mg(s),E_(red )^(o)=-2.370V):}]The cell reaction as written above is spontaneous for the concentrations given: true false