Home /
Expert Answers /
Chemistry /
what-is-the-mathrm-oh-concentration-hint-use-the-k-text-w-relationship-pa744
(Solved): What is the \( \mathrm{OH}^{-} \)concentration? [Hint: Use the \( K_{\text {w }} \) relationship.] ...
![What is the \( \mathrm{OH}^{-} \)concentration? [Hint: Use the \( K_{\text {w }} \) relationship.]
Express your answer with t](https://media.cheggcdn.com/study/f90/f900f498-36f2-4b92-bbd4-e608349259d6/image)

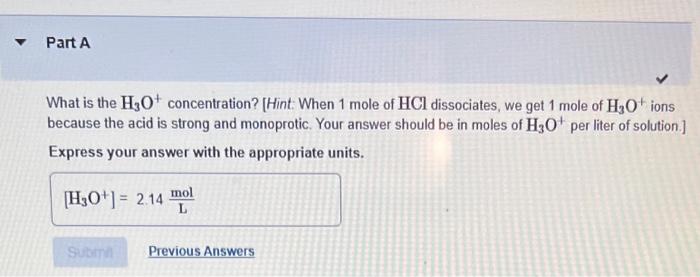
What is the \( \mathrm{OH}^{-} \)concentration? [Hint: Use the \( K_{\text {w }} \) relationship.] Express your answer with the appropriate units.
Suppose \( 1.34 \mathrm{~mol} \) of \( \mathrm{HCl} \) is dissolved in enough water to give \( 625.0 \mathrm{~mL} \) of solution.
What is the \( \mathrm{H}_{3} \mathrm{O}^{+} \)concentration? [Hint: When 1 mole of \( \mathrm{HCl} \) dissociates, we get 1 mole of \( \mathrm{H}_{3} \mathrm{O}^{+} \)ions because the acid is strong and monoprotic. Your answer should be in moles of \( \mathrm{H}_{3} \mathrm{O}^{+} \)per liter of solution.] Express your answer with the appropriate units.
Expert Answer
A). Moles of HCl = 1.34 mol Volume of Solution = 625.0 mL When HCl dissolved in water HCl + H2O ---