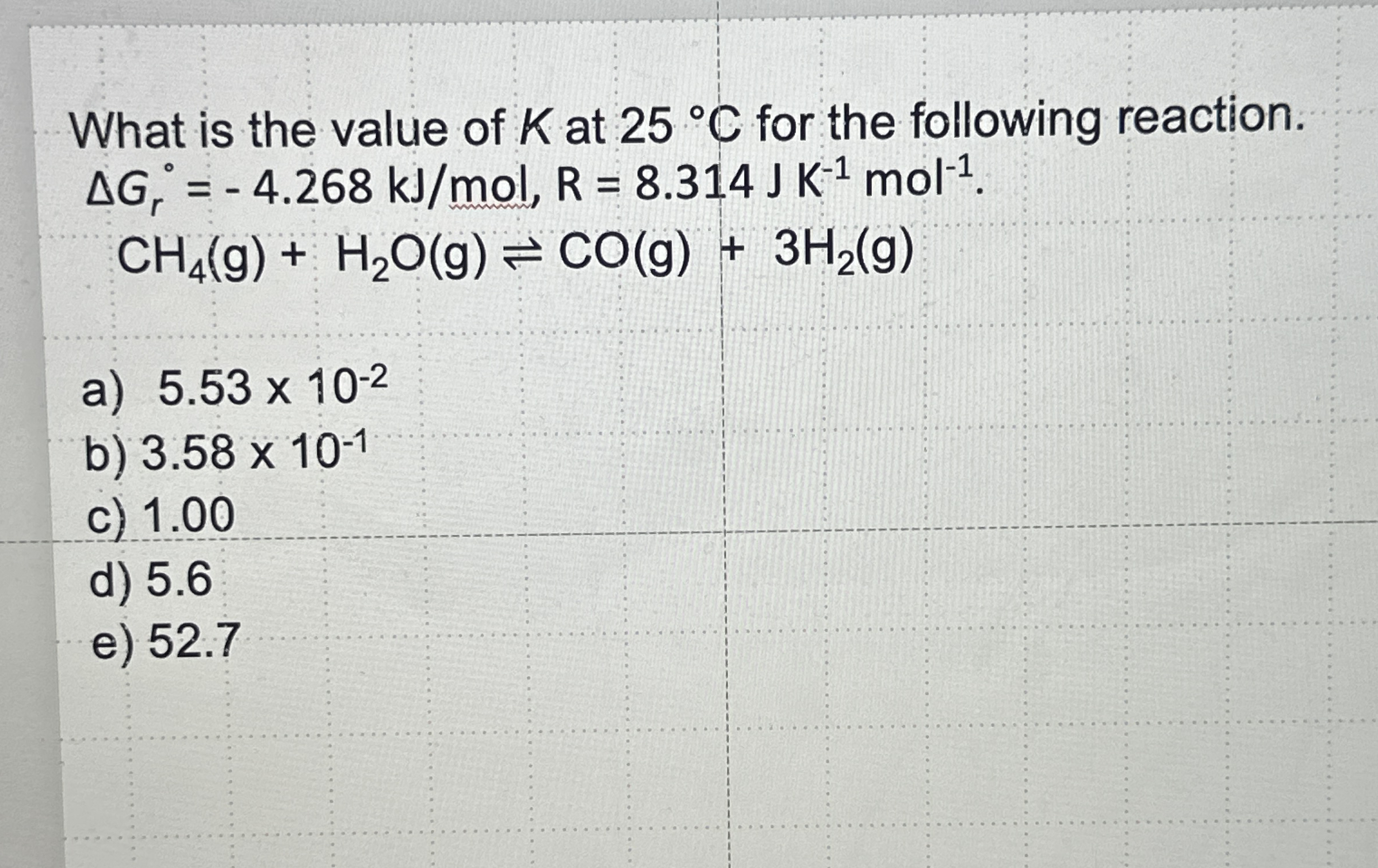Home /
Expert Answers /
Chemistry /
what-is-the-value-of-k-at-25-deg-c-for-the-following-reaction-delta-g-r-0-4-268k-j-m-ol-r-8-pa142
(Solved): What is the value of K at 25\deg C for the following reaction. \Delta G_(r)^(0)=-4.268k(J)/(m)ol,R=8 ...
What is the value of
Kat
25\deg Cfor the following reaction.
\Delta G_(r)^(0)=-4.268k(J)/(m)ol,R=8.314JK^(-1)mol^(-1).
CH_(4)(g)+H_(2)O(g)⇌CO(g)+3H_(2)(g)a)
5.53\times 10^(-2)b)
3.58\times 10^(-1)c) 1.00 d) 5.6 e) 52.7