Home /
Expert Answers /
Chemistry /
what-volume-of-carbon-dioxide-is-produced-when-8-19-mathrm-g-of-calcium-carbonate-reacts-c-pa901
(Solved): What volume of carbon dioxide is produced when \( 8.19 \mathrm{~g} \) of calcium carbonate reacts c ...
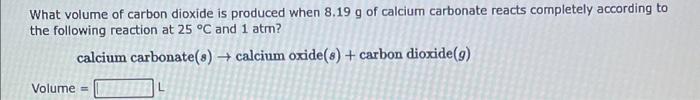

What volume of carbon dioxide is produced when \( 8.19 \mathrm{~g} \) of calcium carbonate reacts completely according to the following reaction at \( 25^{\circ} \mathrm{C} \) and \( 1 \mathrm{~atm} \) ? calcium carbonate \( (s) \rightarrow \) calcium oxide \( (s)+ \) carbon dioxide \( (g) \) Volume \( = \)
How many grams of iron are needed to completely consume \( 51.0 \mathrm{~L} \) of chlorine gas according to the following reaction at \( 25^{\circ} \mathrm{C} \) and \( 1 \mathrm{~atm} \) ? iron \( (\mathrm{s})+ \) chlorine \( (\mathrm{g}) \longrightarrow \) iron(III) chloride ( \( \mathrm{s} \) ) grams iron
Expert Answer
CaCO3 ---> CaO + CO2 number of moles of CaCO3 reacted = mass/molar mass of CaCO3 = 8.19/100 = 0.0819 mol from given chemical equation, moles of CO2 produced = moles of CaCO3 rea