Home /
Expert Answers /
Chemistry /
when-you-add-nacl-or-dextrose-to-mixture-c-do-you-think-these-additives-interact-with-gold-nanopa-pa115
(Solved): When you add NaCl or dextrose to mixture C, do you think these additives interact with gold nanopa ...
When you add NaCl or dextrose to mixture C, do you think these additives interact with gold nanoparticles, excess citrate, or both? a. Explain the interactions you think might be occurring. Saved Normal BI IU X2 | X? III III = for ill III TE (2pts) b. Sketch a picture to support this explanation. Upload a picture of your sketch.
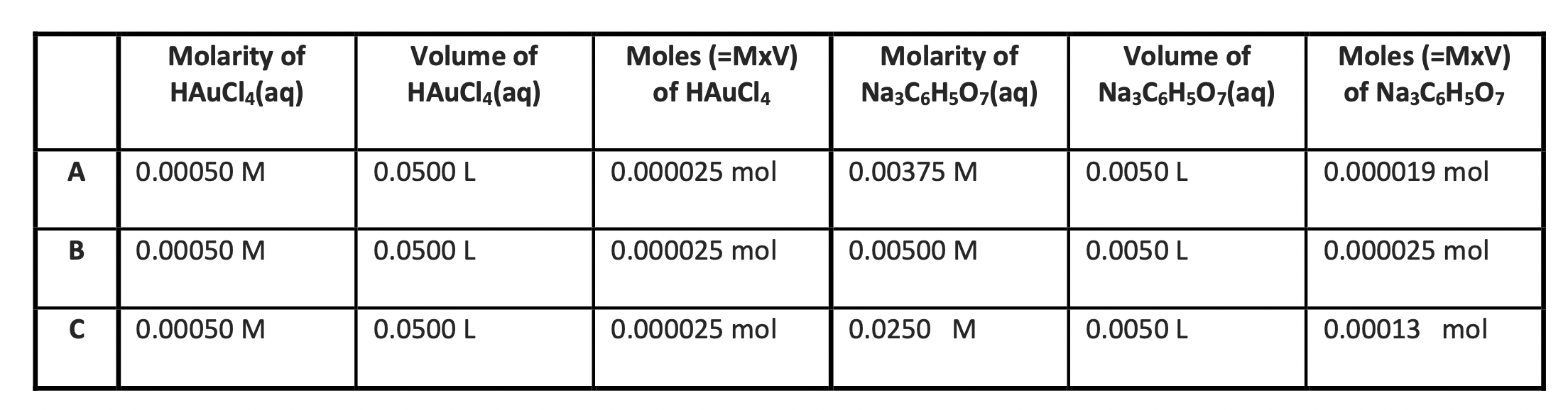
Molarity of HAuCl(aq) Volume of HAuCl(aq) Moles (=MxV) of HAuCl4 Molarity of Na3C6H507(aq) Volume of Na3C6H507(aq) Moles (=MxV) of NazC6H507 A 0.00050 M 0.0500 L 0.000025 mol 0.00375 M 0.0050 L 0.000019 mol B 0.00050 M 0.0500 L 0.000025 mol 0.00500 M 0.0050 L 0.000025 mol C 0.00050 M 0.0500 L 0.000025 mol 0.0250 M 0.0050 L 0.00013 mol
Expert Answer
Adding dextrose (a carbohydrate) or NaCl will have an effect on both species, i. e. on the Au-nanoparticles and on the excess citrate ions that are left from the reaction. The size of these nanopar