Home /
Expert Answers /
Chemistry /
which-of-the-following-reactions-is-spontaneous-at-all-temperatures-begin-array-l-2-mathrm-pa595
(Solved): Which of the following reactions is spontaneous at all temperatures? \[ \begin{array}{l} 2 \mathrm{ ...
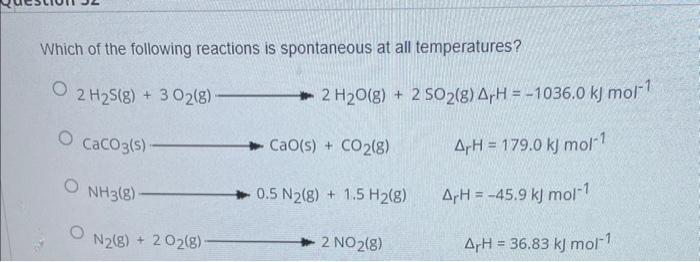
Which of the following reactions is spontaneous at all temperatures? \[ \begin{array}{l} 2 \mathrm{H}_{2} \mathrm{~S}(\mathrm{~g})+3 \mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})+2 \mathrm{SO}_{2}(\mathrm{~g}) \Delta_{\mathrm{r}} \mathrm{H}=-1036.0 \mathrm{~kJ} \mathrm{~mol}{ }^{-1} \\ \mathrm{CaCO}_{3}(\mathrm{~s}) \longrightarrow \mathrm{CaO}(\mathrm{s})+\mathrm{CO}_{2}(\mathrm{~g}) \quad \Delta_{\mathrm{r}} \mathrm{H}=179.0 \mathrm{~kJ} \mathrm{~mol}^{-1} \\ \mathrm{NH}_{3}(\mathrm{~g}) \longrightarrow 0.5 \mathrm{~N}_{2}(\mathrm{~g})+1.5 \mathrm{H}_{2}(\mathrm{~g}) \quad \Delta_{\mathrm{r}} \mathrm{H}=-45.9 \mathrm{~kJ} \mathrm{~mol}^{-1} \\ \mathrm{~N}_{2}(\mathrm{~g})+2 \mathrm{O}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{NO}_{2}(\mathrm{~g}) \quad \Delta_{\mathrm{r}} \mathrm{H}=36.83 \mathrm{~kJ} \mathrm{~mol}^{-1} \\ \end{array} \]