Home /
Expert Answers /
Chemistry /
why-does-the-first-sn1-reaction-have-two-products-excluding-hcl-and-the-second-sn1-reaction-only-h-pa402
(Solved): Why does the first SN1 reaction have two products (excluding HCl) and the second SN1 reaction only h ...
Why does the first SN1 reaction have two products (excluding HCl) and the second SN1 reaction only has one product (excluding HI)?
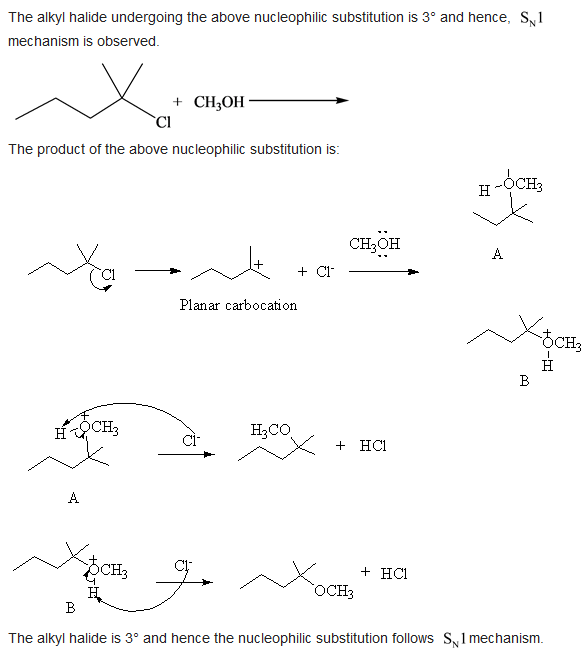
The alkyl halide undergoing the above nucleophilic substitution is \( 3^{\circ} \) and hence, \( \mathrm{S}_{\mathrm{N}} 1 \) mechanism is observed. The product of the above nucleophilic substitution is: Planar carbocation A The alkyl halide is \( 3^{\circ} \) and hence the nucleophilic substitution follows \( \mathrm{S}_{\mathrm{N}} 1 \) mechanism.
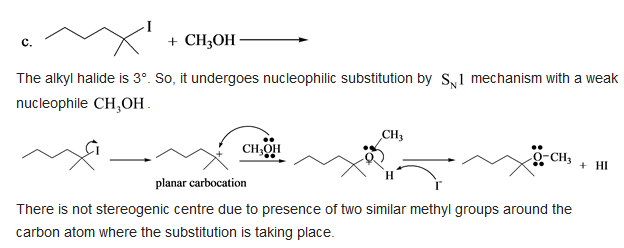
c. The alkyl halide is \( 3^{\circ} \). So, it undergoes nucleophilic substitution by \( \mathrm{S}_{\mathrm{N}} 1 \) mechanism with a weak nucleophile \( \mathrm{CH}_{3} \mathrm{OH} \). There is not stereogenic centre due to presence of two similar methyl groups around the carbon atom where the substitution is taking place.
Expert Answer
The reason for two products in the first reaction is, that