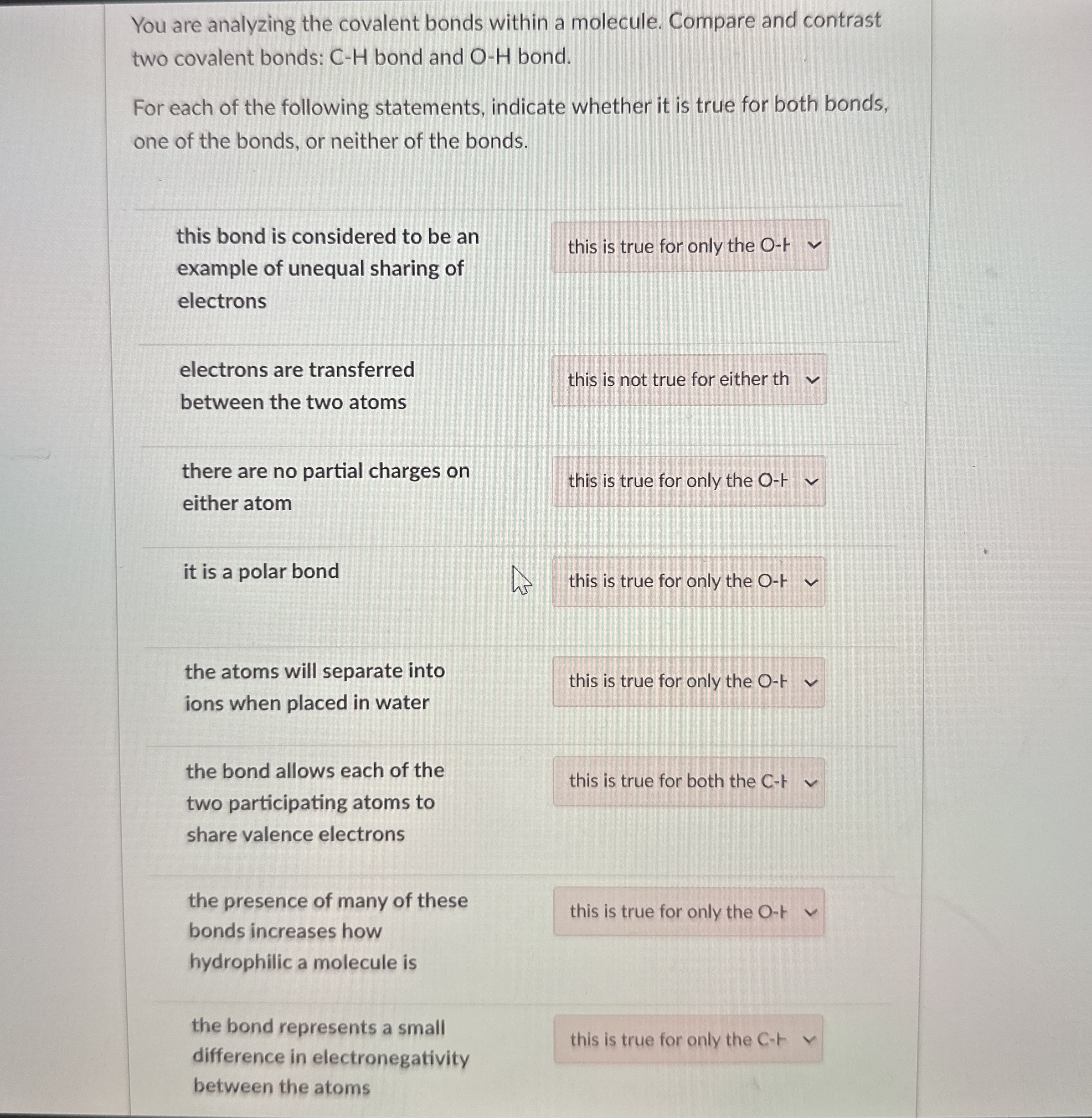
(Solved): You are analyzing the covalent bonds within a molecule. Compare and contrast two covalent bonds: C-H ...
You are analyzing the covalent bonds within a molecule. Compare and contrast two covalent bonds:
C-Hbond and
O-Hbond. For each of the following statements, indicate whether it is true for both bonds, one of the bonds, or neither of the bonds. this bond is considered to be an example of unequal sharing of electrons electrons are transferred between the two atoms there are no partial charges on either atom it is a polar bond the atoms will separate into ions when placed in water the bond allows each of the two participating atoms to share valence electrons the presence of many of these bonds increases how hydrophilic a molecule is the bond represents a small difference in electronegativity between the atoms this is true for only the O-F this is true for only the O-1 this is true for both the C-ト
◻