Home /
Expert Answers /
Chemistry /
zn-s-h2so4-aq-znso4-aq-h2-g-suppose-an-engincer-decides-to-study-the-rate-of-th-pa410
(Solved): Zn(s)+H2SO4(aq)ZnSO4(aq)+H2(g) Suppose an engincer decides to study the rate of th ...
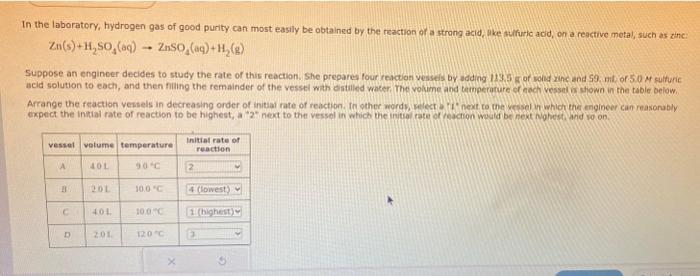
Suppose an engincer decides to study the rate of this reaction. She prepares four reaction verseis by adding 1 ts. 5 ?? of woled zinc and 59 . nity of 50 Af cuifuric acid solution to each, and then filing the remainder of the vessei wath distalled woter, The volume and temperature or cech vesselir stiown in the table below. Afrange the feaction vessels in decreasing order of inital rate of reaction. In pther werds, wilect 7 if next to the vessel vi which the engineer can reasonubly oxpect the initial rate of reaction to be highest, a next to the vessel in which the inital fate of feqction would be mext hiplicst ard so on.
Expert Answer
Given, Mass of Zn = 113.5 g Concentration of HA2SOA4 = 5M Volume of HA2SOA4 = 59 mL = 0.059 LVessel 1: 4 L at 9?CVe